BCRP (breast cancer resistance protein), the most recently discovered member of the ABC transporter superfamily, belongs to the transporters of current interest in the pharmaceutical industry for its recognized involvement in drug-drug interactions. BCRP has two major drug transport functions: first, it limits the distribution of its substrates into organs such as the placenta, testes, brain, and across the gastrointestinal tract (GIT); second, it eliminates the substrates from excretory organs, mediating both biliary and renal excretion, and occasionally direct gut secretion. BCRP also plays a role in the multidrug resistance syndrome of certain cancers, in which efflux transporters are overexpressed and anticancer drugs are expelled out of the cells, resulting in resistance to the treatment. For these reasons, BCRP inhibitors and substrates should be routinely detected within drug candidates during drug development.
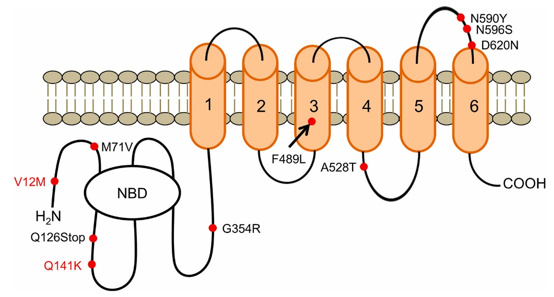 Figure 1. Structure of the BCRP protein.
Figure 1. Structure of the BCRP protein.
The clinical evidence of BCRP inhibition as a mechanism of DDIs with potential safety and efficacy consequences has prompted the International Transporter Consortium to highlight BCRP as an important transporter to evaluate during drug development. Identifying drugs that act as BCRP inhibitors could aid in designing better therapeutic strategies for cancer treatment and will be critical for identifying potential drug-drug interactions.
BCRP Inhibition Assay at Creative Bioarray
Depending on the objective in BCRP pharmacokinetic transport studies, we have established several in vitro assays:
- ATPase assays
- Vesicle assays
- Accumulation assays
- Bidirectional assays
- Uptake assays
References
- Chen L.et al.; Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharmaceutica Sinica B, 2019, 9(4): 659-674.
- Volpe D. A. et al.; Transporter assays as useful in vitro tools in drug discovery and development. Expert Opinion on Drug Discovery, 2015, 11(1): 91-103.
- Lee C. A. et al.; Breast cancer resistance protein (ABCG2) in clinical pharmacokinetics and drug interactions: practical recommendations for clinical victim and perpetrator drug-drug interaction study design. Drug Metabolism and Disposition, 2015, 43(4): 490-509.