Radioligand binding assays have traditionally been the mainstay of drug discovery and drug development. Currently, they are mainly used for membrane-bound molecules, such as G-protein coupled receptors.
Radioligand binding assays are extremely versatile, easy to perform, and can be automated to provide very high throughput. The quality of the data allows the determination of drug affinity, receptor type, receptor distribution, and allosteric interactions. Due to their robustness and sensitivity, radioligand binding assays are considered to be the gold standard for measuring the affinity of ligand binding to its target receptor. There are three experimental types of radioligand binding assays: saturation assay, competition assay and kinetic assay.
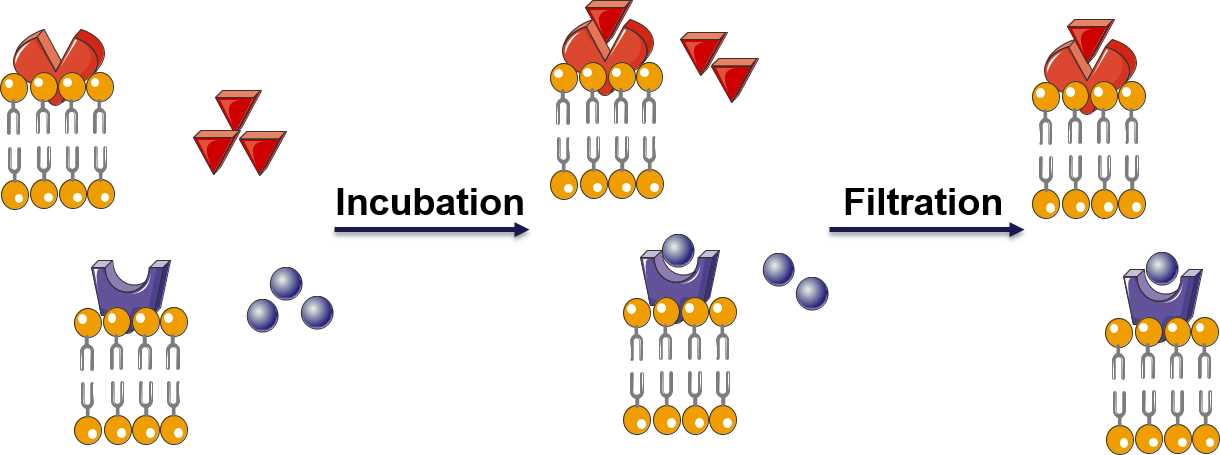 Figure 1. Principle of a receptor-ligand binding assay.
Figure 1. Principle of a receptor-ligand binding assay.
Saturation radioligand binding assays allow determination of the number of binding sites (Bmax) in tissue or cultured cells and the dissociation constant (Kd) of the radioligand to measure affinity. They are carried out as the concentrations of the radioligand increases, and the level of receptor binding can be directly measured. At Creative Bioarray, multiple concentrations (up to twelve) of the radioligand are incubated in triplicate or quadruplicate with either cell or tissue homogenate containing the target receptor of interest.
Competition radioligand binding assays are used to determine the relative affinity (Ki) of a test compound that binds to a receptor site in membrane homogenates or cells. They are performed by incubating a range of concentrations of the unlabeled test compound with a fixed concentration of radioligand and measuring the IC50 of the test compound. At Creative Bioarray, cells or tissue homogenates containing the receptor of interest are incubated together with the radioligand and a range of concentrations of the test compounds (competing). Typically, up to 11 concentrations of the test compounds are evaluated over a three log unit range and triplicate (or quadruplicate) replicates at each concentration.
Kinetic radioligand binding assays are used to determine the association and dissociation rates of radioligands and receptors, providing additional information for optimizing assay conditions.
Association: Cell or tissue homogenate containing the receptor of interest is incubated together with a single concentration of labeled ligand. The specific binding is measured at different times in quadruplicate replicates at each time point.
Dissociation: Cell or tissue homogenate containing the receptor of interest is incubated together with a single concentration of labeled ligand for 120 min. Then, dissociation is induced by adding 10 µM of unlabeled ligand and counted at different time points.
Key Data for Each Report Includes:
- Percent inhibition (mean of replicates)
- Individual values as a percent of control, IC50 or EC50 (calculated from a minimum of 6 concentration testing)
- Ki value (calculated using Cheng-Prussoff equation, and the true Kd of the membrane preparation used for the experiment)
- Hill coefficient (nH)
- Plotted IC50 or EC50 curves
Key Features of Our Radioligand Binding Assay Services:
- Over 100 of targets covered
- IC50 determination of single concentration or dose-response
- A variety of standard panels to choose from
- Majority of membrane preps produced in-house
- >10 scintillation counters capacity
- Assays can be carried out using either radiolabeled antagonists or agonists
- Reliable, quick turnaround time
- High quality data with strict quality control
- Flexible custom services to supplement our off-the-shelf services to meet your needs