Heat shock protein (HSP90) belongs to a family of molecular chaperones which are rapid and abundantly induced by stresses. HSP90 plays a crucial role in stabilizing denatured proteins that have become misfolded or unfolded because of cellular stress and by aiding in their re-folding. So far, many of the proteins identified as HSP90 clients are key components of the oncogenic phenotype involved in controlling many of the hallmarks of cancer.
Inhibiting Hsp90 has the potential to affect all the hallmarks of cancer, making it an exciting potential therapeutic target. HSP90 inhibitors that bind the ATP binding pocket standing on the N-terminal of HSP90 have resulted in degradation of HSP90 client proteins through the ubiquitin proteasome pathway. The natural product of HSP90 inhibitors geldanamycin and radicicol can exert their antitumor function by blocking the intrinsic ATPase activity of HSP90, leading to degradation of HSP90 client proteins. Thus, HSP90 inhibition provides an important pharmacological platform for anticancer therapy.
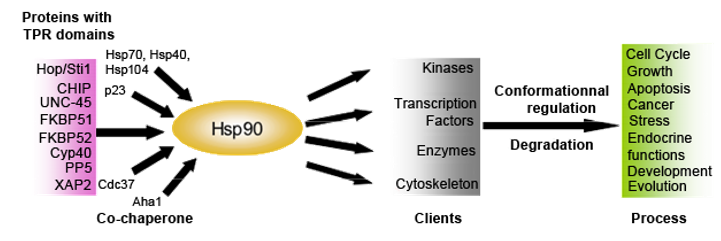 Figure 1. Hsp90 protein partners and clients destabilized by Hsp90 inhibition.
Figure 1. Hsp90 protein partners and clients destabilized by Hsp90 inhibition.
As a pioneer and leader in the drug discovery service, Creative Bioarray has a high level of proficiency in providing HSP90 screening services for customers all over the world. We have developed multiple high-throughput screening (HTS) assays based on a variety of techniques to screen large chemical libraries to identify new Hsp90 inhibitors. Screenings have been developed based on the ability of compounds to:
- Inhibit HSP90 catalyzed ATP hydrolysis;
- Inhibit HSP90-mediated refolding of denatured protein (e.g., luciferase);
- Competitively inhibit the binding of ligand to Hsp90's N-terminal ATP binding domain;
- Deplete cultured cells of HSP90 client proteins.
These assays have identified a large number of potential HSP90 inhibitors. In addition, our team of experts along with our broad services portfolio makes it easy to:
- Screen for targets/ inhibitors using our HSP90α and HSP90β assays
- Select from IC50 determination and single point concentrations
- Perform follow-up studies using the same proteins manufactured in-house
- Get questions answered or project guidance in a time-efficient manner
Creative Bioarray can provide you the comprehensive services and highest quality results in the HSP90 screening and profiling. If you have any special requirements in HSP90 inhibitor screening, please feel free to contact us. We are looking forward to working together with your attractive projects.
References
- Peyrat, Jean François, et al. Inhibitors of the heat shock protein 90: from cancer clinical trials to neurodegenerative diseases. 2011.
- Davenport J. et al. High-Throughput Screen of Natural Product Libraries for Hsp90 Inhibitors. Biology, 2014, 3(1): 101-138.
- Phosphatase Screening Services
- Phosphodiesterase Screening Services
- Poly (ADP-ribose) Polymerase (PARP) Screening Services
- GPCR Screening Services
- Kinase Screening Services
- Transporter Screening Services
- Ion Channel Screening Services
- Nuclear Receptor Screening Services
- Protease Screening Services
- Ubiquitin Screening Services
- High Throughput Screening Services
- High Content Screening Services
- Target-based Screening Services
- Phenotypic Screening Services
- Apoptosis Screening
- Epigenetics Screening Services