GPCR internalization is a key regulatory step in determining receptor activity. It is essential to prevent cells from being subjected to excessive receptor stimulation or prolonged inactivity. If GPCR internalization is inhibited by a ligand, protein mutation, or an abnormal signaling, adverse effects may occur, often resulting in drug tolerance, unwanted side effects, and disease.
The broad applicability of GPCR internalization assays is based on the common phenomenon of GPCR "desensitization" and has been demonstrated by numerous GPCRs. During desensitization, GPCR kinases (GRKs) phosphorylate agonist-activated GPCRs on serine and threonine residues. Arrestin, a cytoplasmic protein, is recruited to the plasma membrane by GRK-phosphorylated GPCRs. The arrestin then uncouples the GPCR from the cognate G protein and targets the desensitized receptors to clathrin-coated pits for endocytosis. In contrast to Ca2+, cAMP and reporter assays, the internalization assay is independent of the associated G protein subclass or individual GPCR intracellular signaling pathway. Thus, there is no need to have prior knowledge of the signaling pathway of a GPCR before using this assay. Internalization assay is particularly useful for de-orphaning GPCRs. With the development of image-based high-content screening (HCS) system, the internalization of GPCRs is now a quantifiable process.
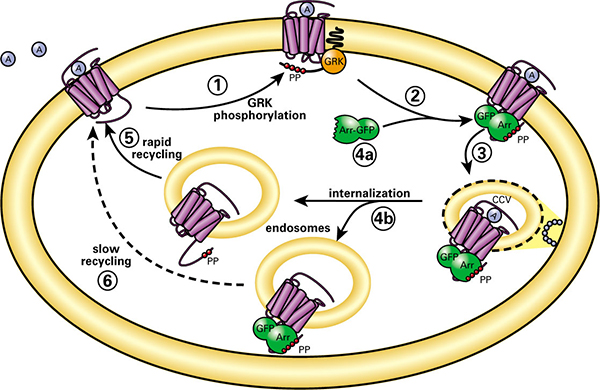 Figure 1. Schematic diagram of the GPCR internalization process.
Figure 1. Schematic diagram of the GPCR internalization process.
Creative Bioarray's GPCR internalization assays are designed to detect compounds or analyze stimuli for their ability to modulate GPCRs activation and the internalization process following. These highly reproducible assays can be performed on our high-content screening platform. If you are interested in a specific GPCR, our Assay Development Department will perform the assay you are interested in for testing your compounds.
Available Standardized GPCRs:
- ADRB2 internalization assay
- BDKRB2 internalization assay
- CB2 internalization assay
- M1 internalization assay
Assay Highlights
- ~100 cell-based assays using a simple one-step protocol applicable to multiple target classes
- Direct functional readout allows for quantitative analysis of GPCR desensitization and recycling as well as uncovering novel classes of compound pharmacology
- Gain-of-signal assays based on chemiluminescence or fluorescence
- No complex data analysis required
References
- Zhang R. et al.; Tools for GPCR drug discovery. Acta Pharmacologica Sinica, 2012, 33(3): 372-384.
- Haasen D. et al.; G protein-coupled receptor internalization assays in the high-content screening format. Methods in Enzymology, 2006, 414: 121-139.
- Receptor Binding Assay
- GTPγS Binding Assay
- Reporter Assay
- cAMP Assay
- Ca2+ Mobilization Assay
- IP3/IP1 Assay
- Label-free Whole Cell Assay
- Receptor Dimerization Assay