Reproductive toxicology is one of the most complicated areas of toxicology research due to the involvement of multiple organs and tissues, different modes of toxicant action and dependence on the endocrine system. In particular, endocrine disrupters represent a significant challenge for experimental toxicology because of their complex effects on signal networks and programming. A variety of in vivo and in vitro developmental and reproductive toxicology (DART) tests are used to predict the safety of new compounds during drug development, and the results provide the basis for internal decision-making.
At Creative Bioarray, our DART capabilities include pharmaceuticals, biologics, vaccines, gene-therapy products, chemicals, and consumer products. Services cover the whole scope of developmental and reproductive toxicology from embryo fetal, postnatal and juvenile stages to the second generation, testing for the potential effects on fertility.
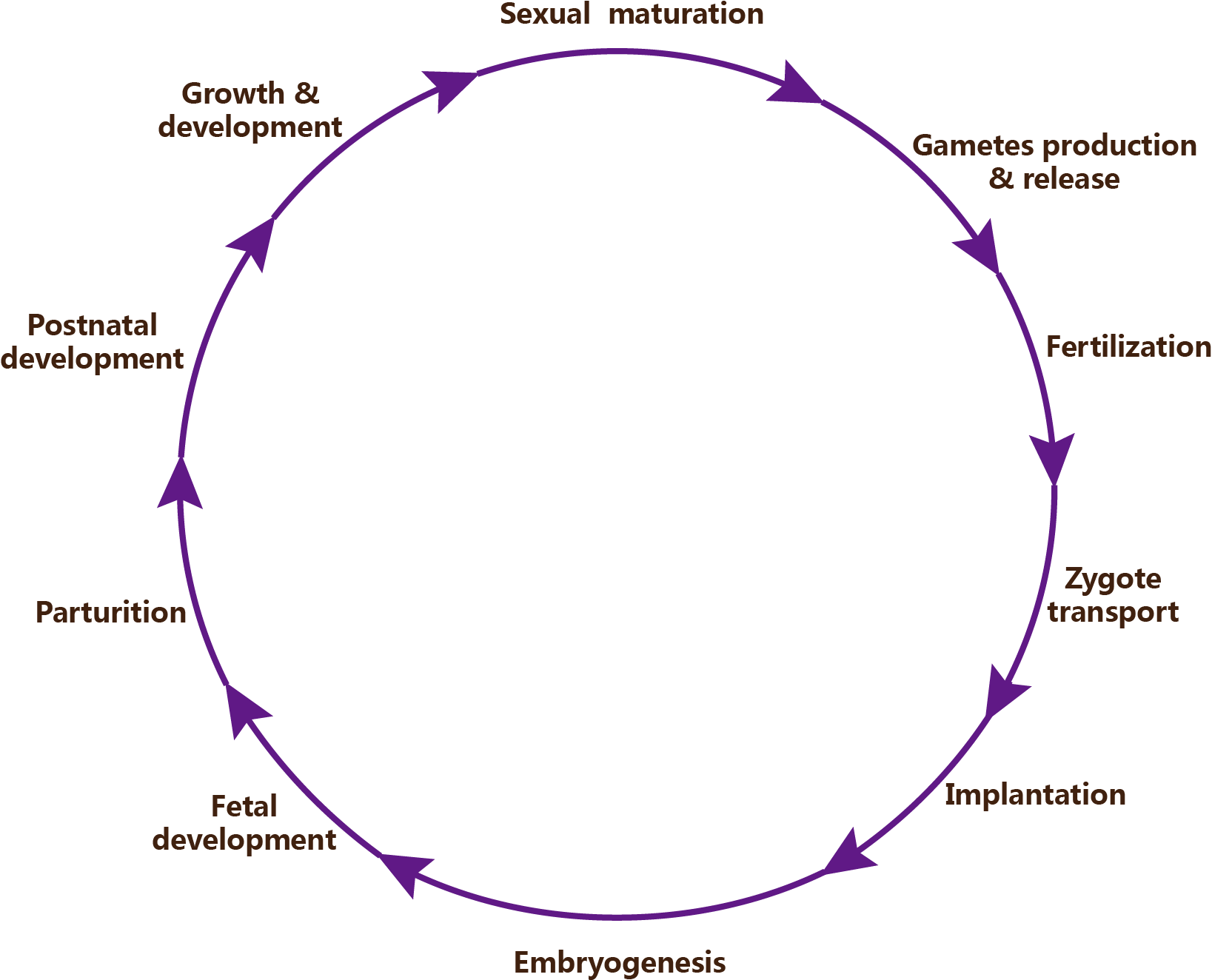 Figure 1. The reproductive cycle of mammalian.
Figure 1. The reproductive cycle of mammalian.
Male and/or Female Fertility
- Fertility and early embryonic development to implantation
- Rat dosed via the clinical route
- Three doses and a control
- Dose males 4 weeks prior to mating, females 2 weeks
- Assess maternal toxicity, development and maturation of gametes, estrous cycles, mating, fertility, and effect on implantation
Embryo Fetal Development
- Rat and rabbit dosed via the clinical route
- Three doses and a control
- Dose during organogenesis (gestation days 6-18)
- Assess ovarian CLs, implantation sites, resorption, dead fetuses, gross fetal malformations and development variations
Prenatal and Postnatal Development
- Female rat dosed via the clinical route
- Three doses and a control
- Dose from gestation day 6 to lactation day 20
- Assess maternal toxicity, pregnancy duration, parturition, lactation, implantation, embryo/fetal changes, stillbirths, growth development, behavior and reproductive changes
One-Generation Reproduction
- Rat of parental generation (F0) dosed via the clinical route
- Three doses and a control
- Dose males 10 weeks prior to mating, females 2 weeks
- Dose both sexes during mating
- Dose females during pregnancy and for the duration of the nursing period
- Assess behavioral changes, food consumption, weight, the number and sex of pups, stillbirths, live births, gross anomalies, pathological changes of reproductive organs
Two-Generation Reproduction
- Rat of first generation (F1) dosed via the clinical route
- Three doses and a control
- Dose both sexes 10 weeks prior to mating
- Dose both sexes 2 weeks during mating
- Dose females throughout pregnancy and up to the weaning of the F2 offspring
- Assess sperm parameters, physical development of the F2 offspring, the age of vaginal opening and preputial separation of F1 weanlings, gross abnormalities, pathological changes of reproductive organs
Creative Bioarray brings together experts in developmental, reproductive, and endocrine toxicology to conduct GLP-compliant toxicology studies. Our DART services are backed by decades of experience in preclinical research and toxicology studies to meet all of your study needs. Contact us to learn more about how we can evaluate the reproductive toxicity potential of your candidates.
- General Toxicology Services
- Immunotoxicology Services
- Inhalation Toxicology Services
- Dermal Toxicology Services
- Ocular Toxicology Services
- Carcinogenic Toxicology Services