Early prediction of drug metabolism and pharmacokinetic properties during drug discovery and development helps streamline the selection of lead compounds that are most likely to become useful drugs. Furthermore, due to time constraints and the large numbers of compounds produced by combinatorial chemistry and high throughput screening, there is a dramatic increase in the need for in vitro assays that can be used to evaluate the biological systems that affect pharmacokinetics of drug candidates both qualitatively and quantitatively. As oral delivery is the most convenient form of administration of pharmaceutical agents in terms of patient compliance, oral administration is preferred when possible. However, intestinal absorption is a powerful barrier that limits the oral bioavailability of many potential new drugs. To address the need for in vitro detection of intestinal permeability of potential new drugs, a variety of approaches have been developed as part of the drug development process. Among these approaches, Caco-2 cell monolayer assay has emerged as one of the standard in vitro tools.
The application of the Caco-2 cell monolayer assay not only facilitates the determination of intestinal permeability, but also enables the identification of specific transporter or efflux proteins and intestinal Phase II drug-metabolizing enzymes. Therefore, this cell-based assay provides valuable information for drug development in an efficient and reproducible manner that is not available from non-biological models and not even available from many other biological models.
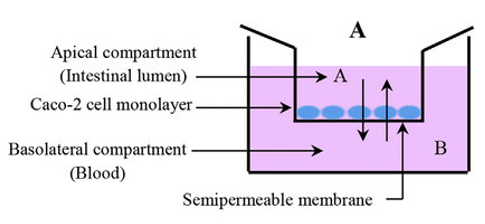 Figure 1. General setup of Caco-2 permeability assay.
Figure 1. General setup of Caco-2 permeability assay.
Caco-2 permeability assay is a part of our portfolio of in vitro ADME screening services. Creative Bioarray delivers consistent, high quality Caco-2 assay data with cost-efficiency that comes from a highly automated approach.
Available Caco-2 Permeability Assay at Creative Bioarray
- Passive permeability in Caco-2
- Evaluation of MDR1 substrate in Caco-2
- Evaluation of BCRP substrate in Caco-2
- Inhibitor assessment in Caco-2 (MDR1 and BCRP)
Deliverables
- The percent recovery of the test compound from the transwell plate with and without cells
- The apparent permeability (Papp) in both directions
- The absorption potential of a test compound
- The efflux ratio [(Papp B to A) / (Papp A to B)]
Reference
- Van Breemen, R. B. et al.; Caco-2 cell permeability assays to measure drug absorption. Expert Opinion on Drug Metabolism & Toxicology, 2005, 1(2): 175-185.