The breast cancer resistance protein (BCRP) functions as an efflux transporter to protect tissues from xenobiotics and harmful metabolites. BCRP is widely expressed on the apical cell membranes of normal cells or tissues, especially in brain tissue, liver and small intestine, mediating the absorption, distribution and elimination of its substrates. The efflux of BCRP requires ATP hydrolysis to allow the molecules to shuttle against the concentration gradient. Activity is altered by the lipid membrane environment, specifically cholesterol content, and may vary between tissues. This pattern of tissue distribution of BCRP confers those drugs (particularly cancer drugs) that are also substrates of BCRP with worse pharmacokinetic properties. Therefore, early recognition of BCRP substrates is essential to optimize oral drug absorption, increase drug bioavailability, and design novel therapeutics aimed at central nervous system conditions and diseases linked to BCRP-mediated cross-resistance issues.
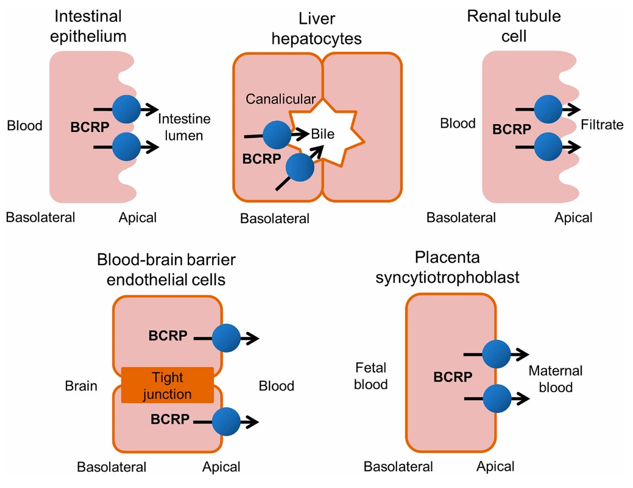 Figure 1. Tissue distribution of human BCRP.
Figure 1. Tissue distribution of human BCRP.
BCRP Substrate Identification Assay at Creative Bioarray
Appropriate in vitro assays for BCRP substrate identification can be divided in two major groups: membrane-based assays and cell-based assays.
- Membrane-based assays
The function of the BCRP efflux transporter and the identification of its substrates are performed by using membranes prepared from cells expressing the BCRP transporter. At Creative Bioarray, there are three membrane-based assays available: ATPase activity assay, membrane vesicular transport assay and photoaffinity labeling assay.
Membrane-based assays have the following advantages: 1) the ability to be used to characterize the effects of xenobiotics on a particular efflux transporter, 2) the ability to be easily employed in a high-throughput mode, 3) the simplicity to be maintained after membrane preparation.
- Cell-based assays
Cell-based assays provide more clear information about the interaction between compounds and BCRP transporters and are used to evaluate the kinetic parameters Km and Vmax of the substrates.
Cell-based assays allow high-throughput screening of compounds due to reduced time consumption and cost when compared with the in vivo assays, which are costly, time-consuming, and ethically limited. In addition, cell culture can be performed in a multi-well plate, which allows for testing of several conditions, concentrations and/or exposure times in a single plate.
References
- Chen L.et al.; Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharmaceutica Sinica B, 2019, 9(4): 659-674.
- Gantner M. E. et al.; Development and validation of a computational model ensemble for the early detection of BCRP/ABCG2 substrates during the drug design stage. Journal of Chemical Information and Modeling, 2017, 57(8): 1868-1880.