The assessment of genotoxicity represents an essential component of the safety assessment of all types of substances. Genotoxicity tests include the measurement of DNA primary damage that can be repaired and is therefore reversible, as well as the detection of stable and irreversible damage (i.e. gene mutations and chromosome aberrations) that is transmissible to the next generation when it occurs in germ cells. Compounds identified as genotoxic in these tests are likely to be carcinogens and/or mutagens, and may eventually induce cancer and/or genetic defects.
The genotoxicity testing of new chemical entities is an integral part of the drug development process and is a regulatory requirement prior to the approval of new drugs. By determing genotoxicity at an early stage in drug discovery rather than during regulatory assessment, the likelihood of late stage failures is reduced.
Creative Bioarray offers a range of genotoxicity testing (non-GLP) services as recommended by regulatory agencies. If there is any positive response in the in vitro study, a follow-up in vivo study of the same endpoint is usually required.
Bacterial Reverse Mutation Test (Ames Test)
In bacterial reverse mutation test, the amino acid-requiring strains of Salmonella typhimurium and Escherichia coli are used to detect the mutation points which may involve substitution, deletion or addition of one or several base pairs. It detects mutations that revert mutations present in the test strains and restore the functional capability of the bacteria to synthesize an essential amino acid. The bacterial reverse mutation test being rapid, inexpensive and easy to perform is commonly used as an initial screening assay for genotoxicity or mutagenicity.
In Vitro Mammalian Chromosomal Aberration Test
The chromosomal aberration test is designed to identify agents that cause structural chromosomal aberrations by using cultured mammalian somatic cells (peripheral blood lymphocytes, Chinese Hamster Ovary cell line (CHO)). After the exposure of cell cultures to the test substance, the cells are treated with a metaphase-blocker, harvested, and stained. The metaphase cells are then analyzed under a microscope for the presence of chromosomal aberrations.
In Vitro Mammalian Cell Micronucleus Test (MNvit)
The micronucleus test detects micronuclei in the cytoplasm of interphase cells. Micronuclei may originate from acentric chromosome fragments (those lacking a centromere) or whole chromosomes that are unable to migrate to the poles during the anaphase stage of cell division. This method identifies the clastogenic and aneugenic activity in cells undergoing cell division during or after exposure to the test substance.
In Vitro Mammalian Cell Gene Mutation Test
This test measures mutation at thymidine kinase (TK), hypoxanthine-guanine phosphoribosyl transferase (HPRT) and at a transgene of xanthine-guanine phosphoribosyl transferase (XPRT). Commonly used cell lines include L5178Y mouse lymphoma cells, the CHO, CHO-AS52 and V79 lines of Chinese hamster cells, and TK6 human lymphoblastic cells. The mutant frequency is determined by seeding known numbers of cells in a medium containing the selective agent in order to detect mutant cells and in a medium without the selective agent in order to determine the cloning efficiency. After an appropriate incubation time, colonies are counted.
In Vitro Comet Test
In vitro comet test, also known as single-cell gel electrophoresis (SCEG) test, is a sensitive method for rapid detection of DNA damage in single cells, including double-strand breaks, single-strand breaks, and alkali labile sites. The comet assay detects the migration of DNA in an agarose gel following electrophoresis, which results in a “head” of intact DNA and a "tail" of fragmented DNA. The percentage of DNA in the tail of the comet is used as a measure of DNA damage.
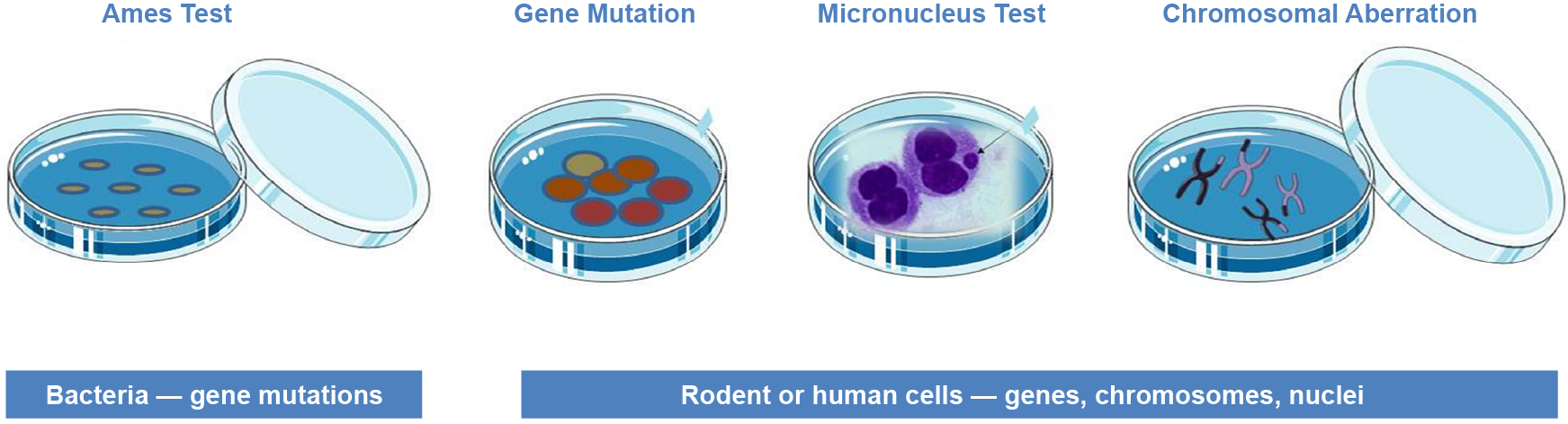 Figure 1. The schematic diagram of in vitro test battery.
Figure 1. The schematic diagram of in vitro test battery.
Key Features of Our Genotoxicity Tests
- Tests meet ICH S2 recommendations
- Tests require a small amount of compound
- Rapid turnaround time (10 business days)
- Objective and consistent data
- Mitochondrial Toxicity Service
- Neurotoxicity Service
- Phototoxicity Testing Services
- Cytotoxicity Testing Services
- Cardiotoxicity Testing Services
- Hepatotoxicity Testing Services
- Nephrotoxicity Testing Services