As G-protein-coupled receptors (GPCRs) can activate several signaling pathways dependent on G-protein subunits they bind to, there have been extensive efforts to develop new, sensitive, and easy-to-use assays to measure second messengers as readout for GPCR activation. Ca2+, serving as a second messenger or charge carrier, mediates the functional responses of a variety of membrane receptors and ion channels. Functional assays measuring changes in intracellular Ca2+ levels have been commonly used to screen for compounds that modulate the activities of target receptors or ion channels.
Ca2+ mobilization is the result of activation of GPCRs binding to specific Gα subunits Gq and Gi. Gq-coupled receptors activate the classical phospholipase C pathway, leading to an increase in inositol triphosphate (IP3) level. IP3 binds to IP3-sensitive calcium channels on the endoplasmic reticulum resulting in the release of calcium from the endoplasmic reticulum into the cytoplasm. Changes in intracellular calcium that reflect GPCR activity can be measured conveniently and accurately using calcium-sensitive dyes.
The Ca2+ mobilization assay is very popular in GPCR screening because of the availability of cell-permeable Ca2+-sensitive fluorescent dyes (such as Fluo-3 and Fluo-4) and automated real-time fluorescence plate readers (such as fluorometric imaging plate reader (FLIPR) and functional drug screening system (FDSS)). FLIPR’s integrated pipetting capabilities allow for ultra-high throughput screening in 384- or 1536-well format, enabling the detection of agonists, antagonists, and allosteric modulators in one assay. The use of fluorescent dyes can also be replaced by the use of Ca2+- sensitive biosensors. Recombinant expression of the jellyfish photoprotein aequorin, which provides an intense luminescent signal in response to elevated intracellular Ca2+ in the presence of a coelenterazine derivative, has also been developed for functional screens of GPCRs.
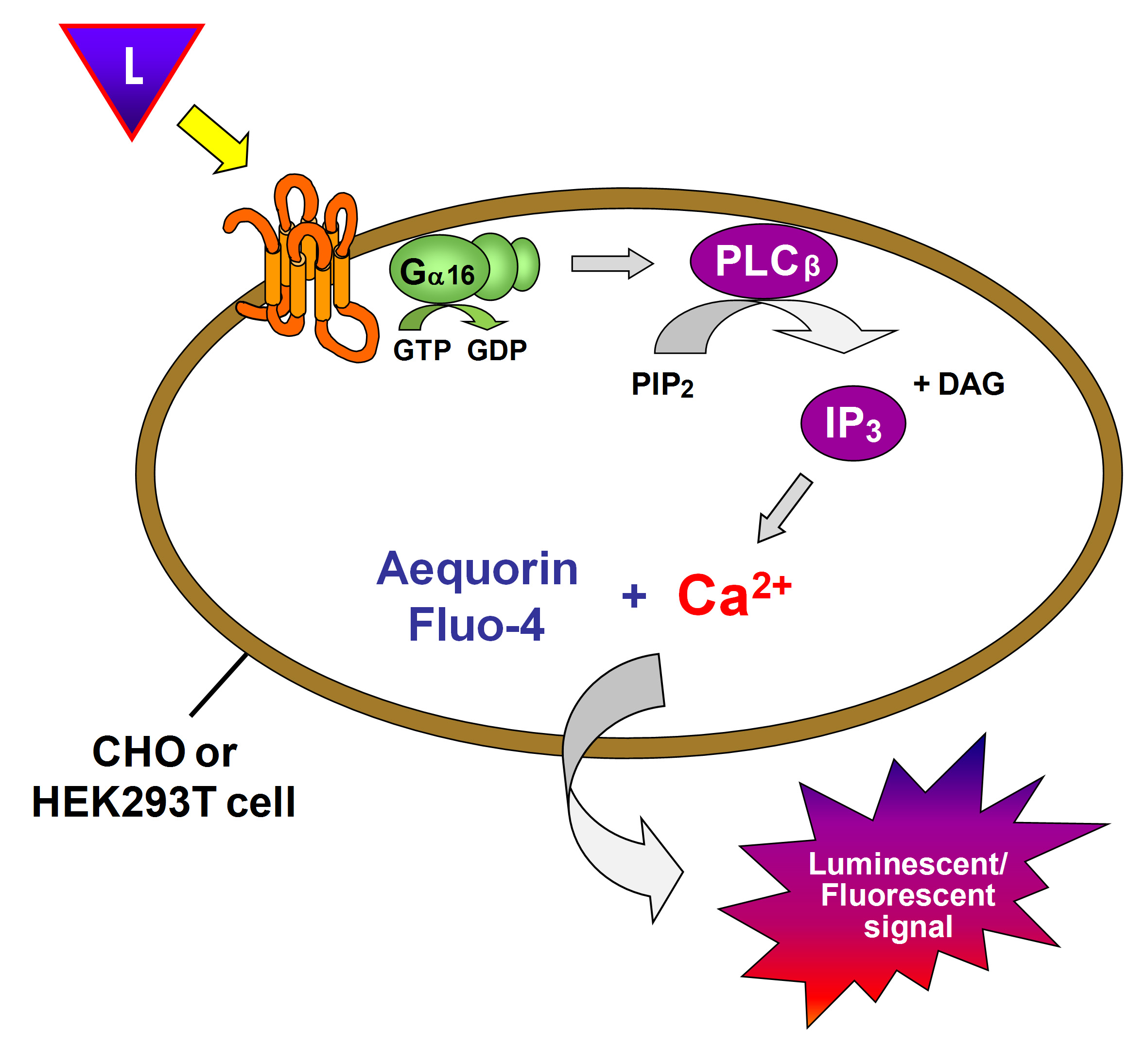 Figure 1. Calcium mobilization assay principle.
Figure 1. Calcium mobilization assay principle.
Ca2+ Mobilization Assay at Creative Bioarray
Ca2+ mobilization assay allows for concentration-dependent analysis of several receptor agonists and antagonists simultaneously, which is very useful in receptor characterization and drug discovery projects. We have a suit of optimized protocols for calcium mobilization experiments with various adherent cells (airway smooth muscle cells, airway epithelial cells, endothelial cells) and non-adherent cells (dendritic cells, monocytes, neutrophils, mast cells, and platelets). These protocols are also applicable to the determination of calcium content in cells overexpressing specific GPCRs.
Features & Benefits
- One step, no wash protocol
- Large assay window and dynamic range with improved signal and performance
- Flexible protocol using adherent or non-adherent cells
- Fewer false positives, due to reduced spontaneous calcium flux and minimal perturbation of cells
References
- Woszczek G. et al.; Ca2+ mobilization assays in GPCR drug discovery. Methods Mol. Biol. 2015, 1272: 79-89.
- Ma Q. et al.; An overview of Ca2+ mobilization assays in GPCR drug discovery. Expert Opinion on Drug Discovery, 2017, 12(5): 511-523.
- Receptor Binding Assay
- GTPγS Binding Assay
- Reporter Assay
- cAMP Assay
- IP3/IP1 Assay
- GPCR Internalization Assay
- Label-free Whole Cell Assay
- Receptor Dimerization Assay