Antibody glycosylation is aimed at selectively regulating specific antibody functions. Glycosylation of proteins is a remarkably dynamic post-translational modification that governs the biology of the majority of cell surface and secreted proteins. The antibody glycan is composed of a biantennary core heptasaccharide composed of a chain of 2 N-acetylglucosamine (Glc NAc) residues, followed by a mannose, followed by a 1,3 and a 1, 6 mannose branching and an additional Glc NAc residue on each mannose.
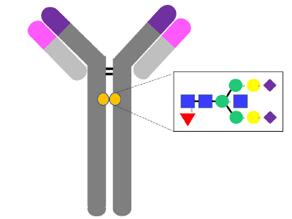 Figure 1. The connection of antibody and its glycosyl. (Galit A. et al. 2018)
Figure 1. The connection of antibody and its glycosyl. (Galit A. et al. 2018)
Antibody Glycosylation in Disease Research
Changes in galactosylation have been widely documented. A breakthrough study points that galactosylation shows unique age-distributions with G0 (no galactose residues) antibodies enriched in the very young and the elderly. The level of galactosylation increases during pregnancy, with a significant improvement in galactosylation and sialylation and decreased bisection that normalizes post-partum. In addition, Fc-glycosylation also varies significantly across geographic regions of the world, and again is dramatically altered in autoimmune and oncological diseases.
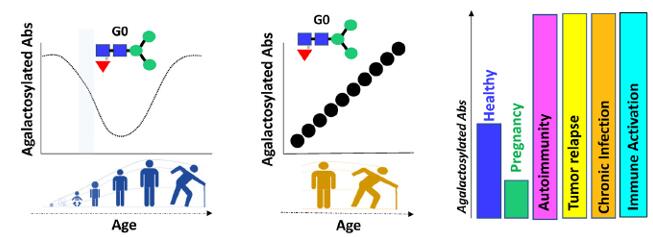 Figure 2. The changes in antibody glycan. (Galit A. et al. 2018)
Figure 2. The changes in antibody glycan. (Galit A. et al. 2018)
Significant differences in antibody glycosylation are observable on antigen-specific antibodies. Moreover, beyond antigen-specific differences, Fc-glycosylation changes are more profoundly altered at the site of disease, where more dramatic differences are observed in IgG Fc-glycosylation in the synovium during rheumatoid arthritic disease and within the cerebrospinal fluid in multiple sclerosis. Yet the functional consequences of these different binding profiles are most clearly illustrated in the context of disease. Besides, the broad impact of intravenous gammaglobulin (IVIG) has clearly illustrated the critical nature of antibody glycosylation on inflammation. Moreover, both in vivo and in vitro data clearly highlight the critical role of IVIG in reduced B cell activation via direct interactions with CD22 during B cell signaling.
Given the intimate role of galactosylation in tuning antibody effector function, including complement activation, it is plausible that alteration in galactose levels is a non-specific mechanism exploited across diseases to rapidly drive immune complex clearance. However, because these complexes persist, chronic immune activation likely also contributes to the pathology of many autoimmune diseases. Antibody glycosylation is influenced by the immune state. Specifically, chronic infections also induce chronic inflammation and are marked by a loss of galactosylated antibodies. Moreover, further studies highlighted that this expansion of galactosylated antibodies occurred in both individuals with progressive HIV infection off therapy as well as in those that spontaneously control HIV infection, despite lower levels of immune activation beyond inflammatory glycan changes. Additional changes were observed within the antigen-specific antibody compartment, associated with enhanced functionality in HIV infections.
Conclusion
Antibody glycosylation profiles have been quite well established in autoimmune diseases and monoclonal antibody therapies, they reveal a few key cross-pathogen themes related to how antibody glycosylation may be impacted by and ultimately shape the immune response to infection. Learning how to direct, control and maintain target antibody glycosylation may provide an alternate means by which next-generation vaccines may drive enhanced control over disease.
References
- Galit A. et al.; Antibody glycosylation in inflammation, disease and vaccination. Seminars in Immunology, 2018, 39:102-110.
- Ackerman ME, Crispin M. et al.; Natural variation in fc glycosylation of HIV-specific antibodies impacts antiviral activity. ClinInvest, 2013, 123(5): 2183-2192.
- Arnold JN, Wormald MR. et al.; The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol, 2007, 25(1): 21-50.