The treatment of primary tumors has seen significant progress yet metastasis continues to be the leading cause of cancer-related death. Metastasis is a complex multi-step process whereby tumor cells spread from a primary tumor to distant sites where they initiate and grow secondary lesions that are resistant to therapy. The effective inhibition of metastasis is currently the ultimate goal of drug development programs in oncology.
The metastatic cascade is a highly inefficient and multi-step process. It is typically conceptualized in five key phases:
- Local invasion and migration;
- Intravasation into vasculature or lymphatics;
- Survival in circulation;
- Extravasation into distant tissues;
- Colonization and outgrowth at the secondary site.
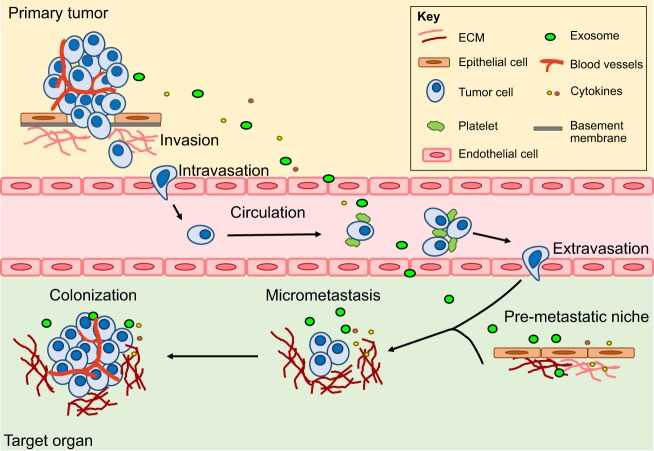 Fig. 1. Metastatic cascade (Gómez-Cuadrado L, Tracey N, et al., 2017).
Fig. 1. Metastatic cascade (Gómez-Cuadrado L, Tracey N, et al., 2017).
Our Metastasis Models platform is designed to help researchers dissect this complex process, by offering them robust clinically relevant in vivo models to screen anti-metastatic and anti-invasive compounds.
Types of Mouse Metastasis Models
| Model Type | Mechanism | Advantages | Limitations |
| Spontaneous Metastasis | Cells implanted orthotopically form a primary tumor that naturally metastasizes. | Recapitulates the entire metastatic cascade; Includes tumor-stroma interactions; High clinical relevance. | Time-intensive; Variable metastatic rate; Potentially lower throughput. |
| Experimental (Directed) Metastasis | Tumor cells are introduced directly into the circulatory system (e.g., IV, intracardiac). | Focuses on late steps (survival, arrest, extravasation, colonization); Highly reproducible; High throughput. | Bypasses early metastatic steps (invasion, intravasation); May not model organ-specific homing accurately. |
| Genetically Engineered Mouse Models (GEMMs) | Metastasis develops spontaneously from autochthonous tumors driven by genetic alterations. | Occurs in an immunocompetent host with an intact tumor microenvironment; Recapitulates human cancer genetics and immunology. | Long timelines; Variable penetrance and latency; Can be costly and genetically complex. |
Available Metastatic Mouse Models
We maintain and utilize a broad portfolio of cell lines and xenografts to establish metastatic models across various tumor types and secondary sites:
Targeted Metastases: Models can be optimized to preferentially develop metastatic lesions in key secondary organs, including:
- Lung Metastasis: Typically established via intravenous injection.
- Bone Metastasis: Often via intracardiac injection, relevant for breast, prostate, and lung cancer.
- Brain Metastasis: Established via intracardiac or carotid artery injection.
- Liver Metastasis: Often established via intrasplenic or portal vein injection.
Tumor Types: Available models cover a range of cancers, including but not limited to:
- Breast Cancer (MDA-MB-231, 4T1, MCF-7)
- Prostate Cancer (PC3, LNCaP)
- Melanoma (B16-F10)
- Lung Cancer (A549, H460)
- Liver Cancer (HCCLM3, HepG2)
- Bowel Cancer (HT-29, HCT-116)
Key Application
Investigating stromal interactions at primary and secondary sites.
Deciphering systemic influences on metastasis.
Elucidating the role of epithelial-mesenchymal transition (EMT).
Therapeutic efficacy testing.
Reference
- Gómez-Cuadrado L, Tracey N, et al. Mouse models of metastasis: progress and prospects. Dis Model Mech. 2017. 10(9):1061-1074.
- Orthotopic Models
- Humanized Mouse Models
- Syngeneic Models
- Cell-based Xenograft Models
- Genetically Engineered Mouse Models (GEMMs)
- Patient-derived Xenograft (PDX) Models